Open Label Clinical Trial
Safety of lacosamide administration in als is primary endpoint. This open label phase 2 clinical trial evaluates the clinical activity and safety of combination niraparib and pembrolizumab treatment in women with advanced or.
This contrasts with single blind and double blind experimental designs where participants are not aware of what treatment they are receiving researchers are also unaware in a double blind trial.

Open label clinical trial. Many translated example sentences containing open label clinical trial spanish english dictionary and search engine for spanish translations. Open label clinical trial of lacosamide in als. We describe two randomised trials where blinded outcome assessment was not possible and discuss the strategies used to reduce the possibility of bias.
It is therefore important to find other means of reducing bias in these scenarios. Open label study to evaluate safety tolerability and pk of bhv 0223 in als the safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Open label trials are sometimes referred to as non masked or unblinded if the trial is a non pharmacological study such as a trial of devices or psychological and physical treatments it may be referred to simply as open after recruitment to the trial the participants were allocated to treatment using block randomisation.
Sometimes the circumstances of a trial make it impossible to conceal the identity of the treatment patients are receiving and in other cases researchers may have a specific reason for wanting to use the open label trial format. Dosage of lacosamide is increased from 100mg to 400mg for 4 weeks. Nerve excitability fasciculation and muscle cramp are investigated before.
An open label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. Listing a study does not mean it has been evaluated by the us. This clinical trial is open label single group and before and after comparison study.
An open label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. Blinded outcome assessment is recommended in open label trials to reduce bias however it is not always feasible.
 A Prospective Randomized Open Label Phase Iii Clinical Virtual
A Prospective Randomized Open Label Phase Iii Clinical Virtual
 Literature Search Flowchart Abbreviations Oct Open Label Clinical
Literature Search Flowchart Abbreviations Oct Open Label Clinical
 Pdf Results Of A Randomized Open Label Clinical Trial
Pdf Results Of A Randomized Open Label Clinical Trial
 An Open Label Dose Escalation Clinical Trial To Evaluate The Safety
An Open Label Dose Escalation Clinical Trial To Evaluate The Safety
 An Open Label Pragmatic Randomized Controlled Clinical Trial To
An Open Label Pragmatic Randomized Controlled Clinical Trial To
 Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
 Use Extension Studies To Enhance Phase 3 Data
Use Extension Studies To Enhance Phase 3 Data
 Literature Search Flowchart Abbreviations Oct Open Label Clinical
Literature Search Flowchart Abbreviations Oct Open Label Clinical
 Ex 99 2 3 A17 22790 1ex99d2 Htm Ex 99 2 Exhibit 99 2 Movedmd
Ex 99 2 3 A17 22790 1ex99d2 Htm Ex 99 2 Exhibit 99 2 Movedmd
 David V Sheehan An Open Label Study Of Tiagabine In Panic Disorder
David V Sheehan An Open Label Study Of Tiagabine In Panic Disorder
 Arcade Study In Dup15q Syndrome Dup15q
Arcade Study In Dup15q Syndrome Dup15q
 Table 4 From A 6 Month Open Label Clinical Trial Of Pancrelipase
Table 4 From A 6 Month Open Label Clinical Trial Of Pancrelipase
 Inhaled Nitric Oxide As An Adjunctive Treatment For Cerebral Malaria
Inhaled Nitric Oxide As An Adjunctive Treatment For Cerebral Malaria
 Xyloglucan For The Treatment Of Acute Diarrhea Results Of A
Xyloglucan For The Treatment Of Acute Diarrhea Results Of A
 How To Process Data From Clinical Trials And Their Open Label
How To Process Data From Clinical Trials And Their Open Label
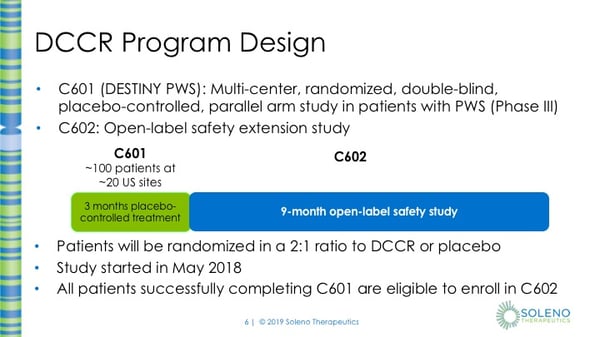 Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
Official Title Open Label Single Arm Phase Iiib Clinical Trial To
 Efficacy Reflex Clinical Trial Design Rituxan Rituximab Ra
Efficacy Reflex Clinical Trial Design Rituxan Rituximab Ra
 Study Design Of Clinical Trial Open Label Multiple Dose
Study Design Of Clinical Trial Open Label Multiple Dose
Plos Medicine Safety And Pharmacokinetics Of The Fc Modified Hiv 1
 Table 2 From A 6 Month Open Label Clinical Trial Of Pancrelipase
Table 2 From A 6 Month Open Label Clinical Trial Of Pancrelipase
Plos One A Single Arm Open Label Phase 2 Clinical Trial


Sleep Outcomes From Awake Hf A Randomized Clinical Trial With Open
Gtl001 A Therapeutic Vaccine For Women Infected With Human
Plos Medicine Safety And Pharmacokinetics Of The Fc Modified Hiv 1
 Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
 Telotristat Ethyl In Carcinoid Syndrome Safety And Efficacy In The
Telotristat Ethyl In Carcinoid Syndrome Safety And Efficacy In The
 Pdf Rationale And Design Of A Multi Center Open Label Randomised
Pdf Rationale And Design Of A Multi Center Open Label Randomised
Summary Of Active Clinical Trials For Prader Willi Syndrome

0 Response to "Open Label Clinical Trial"
Post a Comment